What Is EXCiPACT?
Regulators require excipient users to qualify their suppliers based on GMP/GDP audits and they have indicated that third party auditing of suppliers is acceptable if a creditable certification body issues certificates and audit reports by employing qualified auditors who are demonstrably credible in suitable GMP/GDP standards and in the needs of the pharmaceutical industry. EXCiPACT asbl is a non-profit organisation that owns and manages oversight of such an independent, high quality, third party Certification Scheme available to pharmaceutical excipient manufacturers and distributors worldwide.
IPEC Federation Qualification Form for Pharma Companies - EXCiPACT
Scheme Components
- ISO 9001:2015 standard
- EXCiPACT GMP 2025 standard (2021, 2017, 2012)
- EXCiPACT GDP 2025 standard (2025, 2017, 2012)
- EXCiPACT GWP 2025 standard
- Certification Body competency qualification
- Auditor competency qualification
- Application of EXCiPACT GMP Standard to Pharmaceutical Auxiliary Materials (PAMs) (2023)
- China GMP Annex (2021)
Who Is Certified
Click here for the list of our certificate holders who currently are based in 20+ countries. They range from major international suppliers with multiple sites, to single site, smaller suppliers in Asia, Europe, Mid-East, and N. America. This list is a reliable source regularly used by pharmaceutical companies during their supplier qualification process and to access audit reports and certificates. Our certificate holders and their customers are increasingly enjoying the benefits of EXCiPACT certification. Consistent annual growth is being achieved by our Registered Certification Bodies.
How It Works
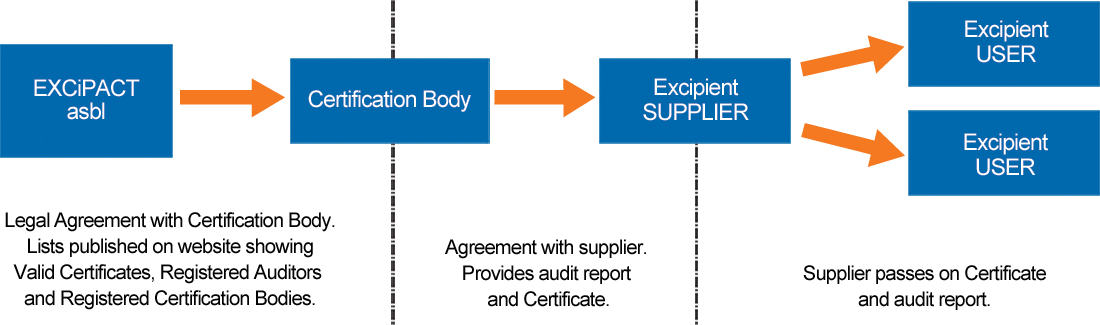
An ISO 9001:2015-approved excipient supplier contracts with an EXCiPACT-approved independent third party Certification Body, agrees the audit scope and standard to be used (GDP, GMP or both), and, if the audit shows compliance, the Certification Body provides them with an EXCiPACT certificate and audit report to share with their customers (users). Certification is valid for three years, includes annual surveillance audits, and as for all Certificate Holders, it is verifiable on the EXCiPACT website.
The Benefits
SUPPLIER COSTS - 3yrs Audit, certificate, surveillance
SUPPLIER SAVINGS - 3yrs Less customer audits, travel, admin
PHARMA COMPANY SAVINGS - 3yrs Less supplier audits, travel, admin
- Saves both supplier and their customers money
- Provided by approved third party Certification Bodies
- Reduces audit burden and cost for supplier and user
- Supported by key regulatory bodies
- Helps excipient users identify qualified suppliers
Latest News
To sign up to receive our latest news alerts direct to your inbox subscribe here
